
A REVIEW OF THE GENUS ACRONICTA OCHSENHEIMER IN SOUTHERN AFRICA, WITH DESCRIPTIONS OF THREE NEW SPECIES
(LEPIDOPTERA, NOCTUIDAE, ACRONICTINAE)
M.KRÜGER
Transvaal Museum, Pretoria
Krüger, M., 2001. A review of the genus Acronicta Ochsenheimer in southern Africa, with descriptions of three new species (Lepidoptera, Noctuidae, Acronictinae). Annals of the Transvaal Museum 38...
The southern African species of the predominantly Holarctic genus Acronicta Ochsenheimer are reviewed. Three species of this genus, which was hitherto represented in subsaharan Africa only by A. transvalica Hampson, 1911, are described as new: Acronicta niveogrisea and A.silvicola from eastern South Africa and Swaziland, and A. vumbae from the eastern highlands of Zimbabwe. All three new species occur in forest habitats and appear to be rare and localized. By contrast, A. transvalica is more widespread and associated with a wide variety of habitats. Information on its distribution is updated, and brief comments are made on the higher classification of the group.
Key words: Lepidoptera, Noctuidae, Acronictinae, new species, forest association, southern Africa
INTRODUCTION AND BRIEF COMMENTS ON HIGHER CLASSIFICATION
Although the subfamily Acronictinae has a cosmopolitan distribution it is poorly represented in the Afrotropics, with most species occurring in temperate regions (Poole, 1989; Scoble, 1992)
Although Acronictinae are cosmopolitan, most species occur in temperate regions, and the subfamily is poorly represented in the afrotropics (Poole, 1989; Scoble, 1992). The moths are greyish, more rarely whitish, cryptically coloured Noctuidae, and the larvae of most species often have colourful secondary setae on the body (Bretherton et al., 1983; Koch, 1984; Scoble, 1992). Although long recognized as a putative natural grouping, some doubts remain as to the monophyly of the Acronictinae. The most recent account of the subfamily is provided by Kitching & Rawlins (1999), who limit the concept of the Acronictinae to Acronicta Ochsenheimer in the broader sense and a few related genera, and conclude that Acronictinae represent a basal and probably paraphyletic taxon.
Acronicta is by far the largest genus within the subfamily, with 166 species currently recognized (Poole, 1989). Although four Palaearctic species reach North Africa (Morocco, Algeria and Tunisia), A. transvalica has so far been the only subsaharan representative.
Although A. transvalica has been reared (label data on Transvaal Museum specimen), no information is available on the early stages or host plants of any Afrotropical Acronicta species, or their host plants.
Classification of the subfamily Acronictinae in the Afrotropical Region
In the first and only comprehensive regional treatment of the family, Gaede (1934) included four genera with a total of 12 species in the Acronictinae (as Acronyctinae). These are Daseochaeta Warren (with 2 species), Thalatha Walker (with 5 species), Craniophora Snellen (with 2 species) and Acronicta Ochsenheimer, with 3 species. Two species, mediovitta Rothschild and pseudobamra Rothschild, have since been transferred from Acronicta to Megalonycta Viette and Tanocryx Viette, respectively. It should be noted that five out of the 12 species listed by Gaede (l.c.) are endemic to Madagascar.
Janse (1937), in revising the southern African fauna, applied a much wider concept of Acronictinae. His Acronictae, placed as a subtribe within a tribe Trifini, roughly correspond to Amphipyrinae + Acronictinae s. str. He included three genera with a single species each, viz. Daseochaeta verbenata (Distant), Craniophora paragrapha (Felder) and Acronicta transvalica Hampson. From a modern viewpoint this approach is unsatisfactory as Amphipyrinae are certainly not monophyletic, and doubt has recently been cast over the monophyly of Acronictinae in a stricter sense as well (Kitching & Rawlins, 1999).
Comments on generic placement of the southern African species
Janse (1937: 131) expressed doubts as to the correct placement of transvalica Hampson in Acronicta, on grounds of differences in male genital structure compared with the type species, Phalaena leporina L., from Europe. These differences include, inter alia, the absence of a harpe and lack of differentiation between the sacculus and dorsal portion of the valvae in transvalica. The genital morphology of the two new species described below suggests that this concern is unfounded: even in these two taxa, which are putative sister-species, a harpe is present in one species only, and the development of a sacculus shows marked differences. The use of such differences to delimit genera would likely require the creation of numerous new entities, with doubtful prospects of improving phylogenetic resolution. It seems advisable therefore to retain Acronicta in a wider sense, based on general similarities of wing pattern and genital structure.
Although Acronicta vumbae spec. nov. is known only from a female, the moth strongly resembles both A. transvalica and many Palaearctic species of Acronicta in wing pattern. It is therefore placed in this genus with a high degree of confidence.
MATERIALS AND METHODS
This study is based on 115 specimens from the collection of the Transvaal Museum, Pretoria, South Africa (TMSA).
Genitalia dissections were made following Robinson (1976). Specimens were stained in a weak solution of alcohol and mercurochrome and slide-mounted in Euparal.
Under Phenology and Habitat associations the main veld types from which the species were recorded are listed, using the numbering and classification of Low & Rebelo (1998). The localities were further plotted on quarter-degree maps supplied by the National Botanical Institute. A Gazetteer at the end of the paper lists all localities from which specimens were examined.
ACRONICTA Ochsenheimer, 1816
Acronicta Ochsenheimer, 1816, Schmett. Eur. 4: 62. Type species: Phalaena leporina Linnaeus, 1758, Syst. Nat. (Edn 10) 1: 510, by subsequent designation by Curtis, 1826, Br. Ent. 3: 136 [Europe] (Nye, 1975).
Synonymy (after Poole, 1989):
Triaena Hübner, 1818
Hyboma Hübner, [1820] 1816
Jochaera Hübner, [1820] 1816
Pharetra Hübner, [1820] 1816 (preoccupied)
Arctomyscis Hübner, [1820] 1816
Apatele Hübner, 1822
Acronycta Treitschke, 1825 (unjustified emendation of Acronicta Ochsenheimer, 1816)
Cometa Sodoffsky, 1837 (unnecessary replacement name for Acronycta Treitschke, 1825)
Semaphora Guenée, 1841 (junior objective synonym of Triaena Hübner, 1818)
Semotophora Agassiz, [1847] (unjustified emendation of Semaphora Guenée, 1841)
Microcoelia Guenée, 1852
Megacronycta Grote, 1873
Lepitoreuma Grote, 1873
Eulonche Grote, 1873
Plataplecta Butler, 1878
Mastiphanes Grote, 1882
Viminia Chapman, 1890 (subjective replacement name for Pharetra Hübner, [1820])
Cuspidia Chapman, 1890
Pseudepunda Butler, 1890
Tricholonche Grote, 1896
Philorgyia Grote, 1896
Chamaepora Warren, 1909
Subacronicta Kozhantschikov, 1950
Key to the Acronicta species of southern Africa
1. Moths greyish (Figs 1, 2) ..... 2
- Moths whitish (Figs 3, 4) ..... 3
2.(1) Median area of forewings without green dusting; basal streak short and bold, not reaching beyond basal line (Fig. 1). Widespread in eastern part of South Africa, with single records from Zimbabwe and southern Mozambique ..... A. transvalica Hampson
- Median area of forewings with faint green dusting; basal streak fine, extending across wings (Fig. 2). Eastern highlands of Zimbabwe (Vumba Mts.); probably Zambia (Solwezi)
..... A. vumbae spec. nov.
3.(1) Moths with basal and postmedian line on forewings fairly distinct; tornal streak very short (Fig. 3). Eastern Cape and KwaZulu-Natal provinces of South Africa, in inland forests
..... A. niveogrisea spec. nov.
- Moths with basal and postmedian line on forewing indistinct; tornal streak long (Fig. 4). KwaZulu-Natal province of South Africa, Swaziland, in coastal forests
..... A. silvicola spec. nov.
Distribution and habitat associations of Acronicta transvalica
Acronicta transvalica Hampson, 1911; Fig. 1, Map 1
Fig1: 1 Acronicta transvalica; 2 A. vumbae; 3 A. niveogrisea; 4 A. silvicola. Scale bars in mm.
Acronicta transvalica Hampson, 1911: 436 (as Acronycta). Type locality: [South Africa, Mpumalanga]: Transvaal, Barberton, Noord Kap.
Acronicta transvalica Hampson; Gaede in Seitz, 1934: 31; Janse, 1937: 131; Poole, 1989: 30.
This species has a far wider distribution than its southern African congeners. In addition to Noord Kaap [now Noordkaap], Janse (1937) gives Eshowe (KwaZulu-Natal), Pretoria (Gauteng), Bloemfontein (Free State), Stellenbosch (Western Cape Province) and Kimberley (Northern Cape Province) as further localities, which suggests that this species is widely distributed.
Distribution (Map 1). From the Western Cape Province across the eastern part of South Africa, especially in the former Transvaal. There are also single records from Zimbabwe and southern Mozambique.
Habitat associations. Throughout its range Acronicta transvalica occurs in more than a dozen veld types falling into the Forest, Savanna, Grassland and Fynbos biomes (Low & Rebelo, 1998).
Material examined. 76 % and 24 &. 100 Pretoria (TMSA).
Localities. South Africa, Western Cape Province: Cape Town (4); Hout Bay (3); Seven Weeks Poort (2); Kogelberg (1); Stellenbosch (2); Jonkershoek (3); Paarl (9); Paarlberg (1); [Farm] Groenkol, Clanwilliam District (1); Pakhuis Pass, Clanwilliam Distr. (1); Algeria Forestry Station, Clanwilliam Distr. (4); Worcester (7); Fish Hoek (3); Bettys Bay (1); De Hoop, Bredasdorp Distr. (5); Wilderness (1). Eastern Cape Province: Buffalo Pass near East London (1); Beacon Bay (4); Umtata (1); Aliwal North (1). Free State: Farm Abel 52, Parys Distr. (1); Bloemfontein (3); Farm Italy, Ladybrand Distr. (1); Oranjekrag, H.F. Verwoerd [now Gariep] Dam (3). KwaZulu-Natal: Eshowe (1); Muden (1), Weenen (1). Mpumalanga: Barberton (2); Fourteen Streams, Barberton Distr. (1); Krokodilpoort Mts., 28 km SE Nelspruit (2); Blyde River (1); Skukuza (1); Malelane (1). Gauteng: Pretoria (5); Suikerbosrand (2). Northern Province: Waterberg (1); Tshakoma, Zoutpansberg (1); Louis Trichardt (1); Naboomspruit (1); Penge, Lydenburg Distr. (2); Percy Fyfe Nature Reserve, Potgietersrus Distr. (2); Limburg, Potgietersrus Distr. (1); Venda, Mutale (2); Wylies Poort (1). Swaziland: Mpisi (3); Mlawula (1); Bulungu Mts (2). Zimbabwe: Christon Bank (1). Mozambique: Bela Vista (1).
Descriptions of new species
1. Acronicta vumbae spec. nov., Fig. 2; Map 2
TYPE MATERIAL. Holotype female, [Zimbabwe]: Laurenceville, Vumba, S[outhern] Rh[odesia], 6.-12.III.1964 (Vári & van Son).- (TMSA).
Fig 2: Map 1
DESCRIPTION: adult female (Fig. 2). Slightly more robust than A. transvalica, its putative sister species. Antennae filiform, grey, tapering in apical third. Labial palpi short, approximately diameter of eyes, and appressed; vestiture of a uniform blackish-brown. Ground colour of forewings whitish, evenly suffused with medium grey, and peppered with darker grey scales. A faint green sheen present in parts of median and postmedian area. Basal, median and postmedian lines present but indistinct; reniform, orbicular and claviform macula equally present but difficult to discern. A fine, curved, transverse black line encircling two small rounded areas below stigmata extending from wing base to above tornus. Hind wings only lightly suffused with grey, notably over postmedian area. Discal spots and postmedian line present but rather indistinct. Cilia on both pairs of wings indistinctly chequered. Underside of wings whitish suffused with grey, especially on forewings, and markings similar to upper side of hind wings, i.e. with an indistinct discal spot and postmedian line present. Vestiture of body concolorous with forewings.
Forewing length. 17 mm (holotype).
DIAGNOSIS. In habitus this moth closely resembles several Palaearctic species of Acronicta, such as A. aceris (Linnaeus, 1758) or A. auricoma ([Denis & Schiffermüller], 1775). The species somewhat resembles A. transvalica but is larger and lacks the prominent tornal streak characterizing that species.
PHENOLOGY AND HABITAT ASSOCIATIONS. Adults have been collected in March (and August, if the Zambian specimen is conspecific). Although the moth fauna of the Vumba Mountains has been comparatively well sampled, only one specimen of A. vumbae was available for study, possibly due to its late appearance.
DISTRIBUTION (Map 2). Eastern highlands of Zimbabwe and probably Zambia (see below).
FURTHER MATERIAL. One specimen in the Transvaal Museum collection lacking metathorax with hind wings and abdomen, but probably a female based on the structure of the antenna, with the following label data: [Zambia], N[orth] W[est] Rhodesia, Solwezi, VIII.1917 (H.C. Dollman), 1919-79.
ETYMOLOGY. Named after the type locality, the Vumba Mountains in the eastern highlands of Zimbabwe.
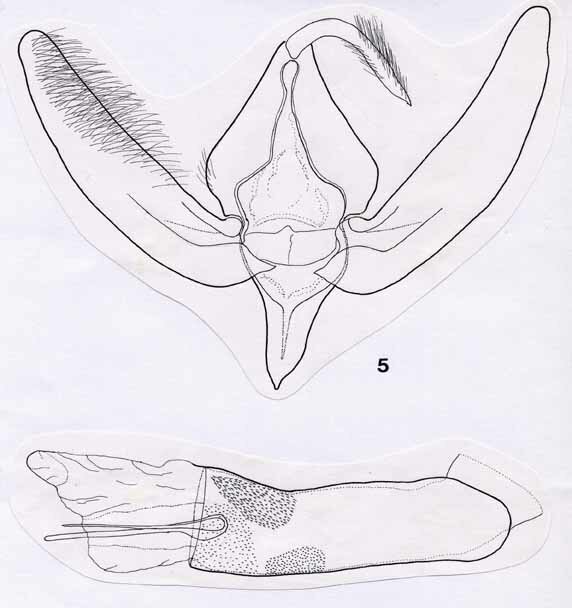
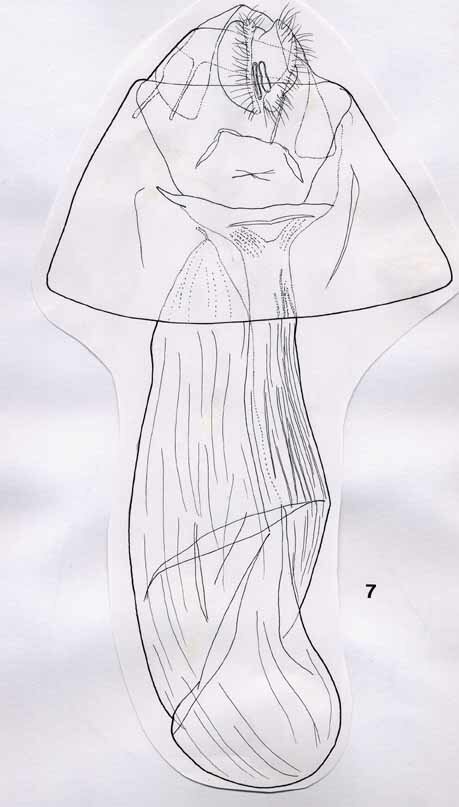
2. Acronicta niveogrisea spec. nov., Figs 3, 5, 7, Map 2
TYPE MATERIAL. Holotype male, [South Africa, Eastern Cape Province]: Hogs Back, Cape, 14.II.1978 (N.J. Duke).- (TMSA).
Paratypes (2 males, 1 female). [South Africa, Eastern Cape Province]: 1 male, same data as holotype (dissected, TM Lep. Heter. Genitalia slide No. 13723). [KwaZulu-Natal]: 1 male, Natal, Nkandla Forest, 27.III.[19]82 (N.J. Duke); 1 female, S[outhern] Africa, Natal, Ngome State Forest, 27°48'S 31°25'E, 18.-22.I.1993 (Krüger & Dombrowsky) (dissected, TM Lep. Heter. Genitalia slide No. 13686).- (TMSA).
Fig3: Map2
DESCRIPTION: adult (Fig. 3). Robust moths; female slightly larger than male. Antennae brown, serrated in both sexes, but rami quite short in male and even shorter in female. Labial palpi short, ascending, approximately 1.5 times diameter of eyes. Scaling on palpi black laterally, whitish mixed with grey in other areas. Wings broad in both sexes. Ground colour of forewings white but wings appearing light grey due to darker grey dusting. Basal and postmedian line well developed, double, but not conspicuous. The inner line basad, and the postmedian line distad finely edged with black. Orbicular and reniform maculae present and fairly large, but poorly delimited and inconspicuous; claviform not discernible. Area between maculae and costa occupied by a prominent blackish-grey mark. Basal streak black, well developed and extending to basal line; tornal streak also black but very short. Hind wings of ground colour, with some faint grey dusting along termen, the latter more strongly developed in female. Discal spots and a strongly convex postmedian line hardly visible. Underside silvery white, glossy. Forewings strongly suffused with brownish-grey, the same suffusion tracing the venation. On hind wings, postmedian line and discal spots prominently developed. Vestiture of body whitish-grey, tegulae laterally demarcated by black.
Forewing length. 17-18 mm (males) (n = 3); 19 mm (females) (n = 1).
Male genitalia (Fig. 5). Uncus long and curved, terminating in a fine hook, and bearing long, recurved setae. Gnathos absent. Tegumen strongly developed, hood-like. Vinculum markedly narrower, strongly tapering towards tip, with v-shaped contours. Valvae long and narrow, recurved and of approximately equal width throughout, only gently tapering towards apex. Most of surface finely setose. Basal half of valvae indistinctly differentiated into costa and sacculus, separated by a narrow membranous area. Clasper absent. Juxta poorly defined, somewhat crescentic. Aedeagus massive relative to size of genital capsule, surface posteriorly with three patches formed by minute denticles. Vesica bearing two long, needle-like cornuti arising from a bulbous base.
Female genitalia (Fig. 7). Large and robust. Papillae anales crescentic in ventral view, with densely setose rim. Both pairs of apophyses short and stout. Bursa copulatrix pipe-shaped, with a long, stem-like and well-sclerotized ductus bursae, widening strongly a short distance below the ostium. Corpus bursae elongated, tear-shaped, with ductus seminalis branching off posteriorly; corpus joined by ductus bursae in anterior half. Wall of both ductus and corpus bursae ribbed; signum absent.
 |
 |
DIAGNOSIS. This moth resembles A. silvicola which is treated below but are more robust and may be readily recognized by the much shorter tornal streak on the forewings. In the male genitalia, the species is characterized by the absence of a clasper on the valvae.
PHENOLOGY AND HABITAT ASSOCIATIONS. Adults have been collected in January-March. Unlike A. silvicola, which occurs in tropical and subtropical coastal forests, A. niveogrisea is probably restricted to temperate moist inland forests (Veld type 2, Afromontane Forest, in Low & Rebelo, 1998).
DISTRIBUTION (Map 2). Eastern Cape and KwaZulu-Natal provinces of South Africa.
ETYMOLOGY. From Latin niveus (-a, -um), snowy white, and griseus (-a, -um), grey, referring to the colouration of the moth.
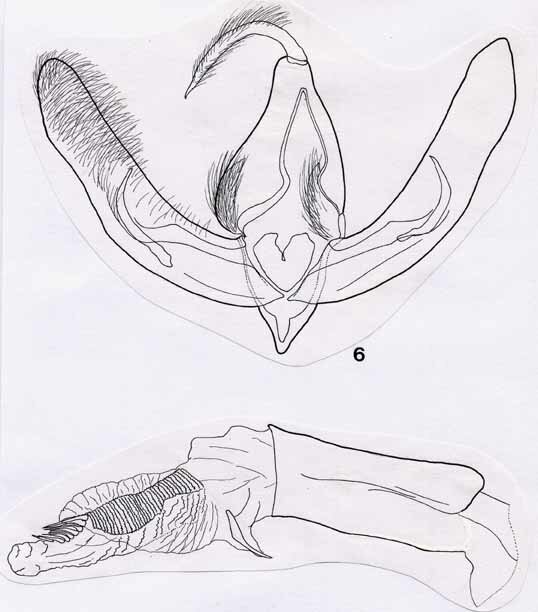
3. Acronicta silvicola spec. nov., Figs 4, 6, 8, Map 2
TYPE MATERIAL. Holotype male, [South Africa, KwaZulu-Natal]: Ngoya Forest, Mtunzini Distr[ict], 19.-21.III.1968 (Potgieter & Goode).- (TMSA).
Paratypes (6 males, 1 female). [South Africa, KwaZulu-Natal]: 2 males, 1 female, same data as holotype (female dissected, TM Lep. Heter. Genitalia slide No. 13786); 1 male, Durban, ex coll. Clark, A.J.T. Janse; PR [meaning unclear]; 1 male, Dukuduku, Nat[al], 22.-24.III.1968 (Potgieter & Goode) (dissected, TM Lep. Heter. Genitalia slide No. 13689); 1 male, ibidem, dated 27.-29.IX.1974 (J. & H. Potgieter). SWAZILAND: 1 male, Jilobi Forest, Lubombo Mtns, 09.III.[19]96 (N.J. Duke).- (TMSA).
DESCRIPTION: adult (Fig. 4). Less robust than the preceding species; female larger than male, with broader wings. Antennae of both sexes monopectinate, but appearing serrated but to shortness of rami; vestiture white, mixed with grey scales. Labial palpi short, ascending, approximately 1.5 times diameter of eyes. Scaling on palpi blackish-grey laterally, whitish mixed with grey in other areas. Ground colour of forewings chalk-white, lightly dusted with pale grey. Basal, median and postmedian lines present, pale grey, but not conspicuous. A pale grey subterminal fascia extending from subapical macula (see below) to tornus. Orbicular and reniform stigma white, inconspicuous to obsolete; claviform absent. Area between stigmata and costa occupied by a grey mark, delimited by black. A grey subapical macula also present, though smaller and less prominent. Basal streak black, well developed and extending to basal line; tornal streak also black, approximately twice the length of basal. Hind wings of ground colour, with some faint grey dusting along termen and tracing venation, the latter more strongly developed in female. Discal spots and postmedian line present but faint. Cilia of both wings white, unevenly chequered with grey. Underside silvery white, glossy, and with light grey suffusion. Discal spots and postmedian line present; in strongly marked specimens the forewing macula above stigmata also visible. Vestiture of body whitish, mixed with grey scales.
Fore wing length. 15-19 mm (males) (n = 8); 20 mm (females) (n = 1).
 |
 |
Male genitalia (Fig. 6). Uncus long and curved, terminating in a fine hook, and bearing long, recurved setae. Gnathos absent. Tegumen strongly developed, hood-like; basis bearing two elliptical, densely setose sclerites. Vinculum markedly narrower, with v-shaped contours. Valvae long and narrow, recurved and of approximately equal width throughout; surface finely setose. Basal half of valvae differentiated into costa and sacculus, separated by a membranous area. Clasper slender and curved, somewhat sickle-shaped. Juxta heart-shaped. Aedeagus massive relative to size of genital capsule; vesica bearing a densely packed subapical series of approximately ten needle-like cornuti, as well as a larger cornutus in an anterior position.
Female genitalia (Fig. 8). Large and robust. Papillae anales crescentic, with strongly setose rim. Apophyses short and stout, arising from broad base, then tapering rapidly. Sterigma crescentic. Ductus bursae short and spindle-shaped, well sclerotized. Corpus bursae very large, broadly triangular, ductus seminalis branching off lateral extrusion near base. Bursal wall membranous, without signum.
DIAGNOSIS. Similar to Acronicta niveogrisea described above but less robust and readily distinguished from it by the much longer tornal streak (compare Figs 3 and 4). In the male genitalia, A. niveogrisea is characterized by the presence of a clapser on the valvae, which is absent in A. niveogrisea. Confusion with other southern African noctuids should not occur.
PHENOLOGY AND HABITAT ASSOCIATIONS. Adults have been collected from September to March, with most records from the latter month. The species inhabits tropical and subtropical coastal forests (present as islands of veld type 3, Sand Forest, within veld type 23, Coastal Bushveld-Grassland, in Low & Rebelo, 1998).
DISTRIBUTION (Map 2). Along the eastern coast of southern Africa between 26° and 32°S. Known localities are near the coast, unlike in A. niveogrisea, which occurs further inland.
FURTHER MATERIAL. [South Africa, Eastern Cape Province:] 1 male, Port St. Johns, 9.I.1971 (D.M. Kroon). This specimen was excluded from the type series because of its poor condition.
ETYMOLOGY. From Latin silva (-ae), a forest, and colo, to inhabit: from the species habitat.
GAZETTEER
SOUTH AFRICA
Western Cape Province
Algeria Forestry, Clanwilliam Distr. 32°23'S 19°03'E
Bettys Bay 34°22'S 18°55'E
Cape Town 33°55'S 18°25'E
De Hoop, Bredasdorp Distr. 34°26'S 20°27'E
[Farm] Groenkol, Clanwilliam Distr. 32°06'S 18°42'E
Fish Hoek 34°08'S 18°26'E
Hout Bay 34°02'S 18°21'E
Jonkershoek 33°58'S 18°55'E
Kogelberg Nature Reserve 34°14'S 18°52'E
Paarl 33°44'S 18°58'E
Paarlberg see Paarl
Pakhuis Pass, Clanwilliam Distr. 32°09'S 19°00'E
Seven Weeks Poort 33°22'S 21°25'E
Stellenbosch 33°56'S 18°51'E
Wilderness 33°59'S 22°35'E
Worcester 33°39'S 19°26'E
Eastern Cape Province
Aliwal North 30°42'S 26°42'E
Beacon Bay 33°01'S 27°58'E
Buffalo Pass near East London 33°05'S 27°49'E
Hogsback 32°06'S 27°01'E
Port St. Johns 31°38'S 29°33'E
Umtata 31°06'S 28°47'E
Free State
Bloemfontein 29°07'S 26°13'E
Farm Abel 52, Parys Distr. 26°54'S 27°35'E
Farm Italy, Ladybrand Distr. c. 29°15'S 27°28'E
Oranjekrag, H.F. Verwoerd [now Gariep] Dam 30°36'S 25°29'E
KwaZulu-Natal
Dukuduku Forest 28°24'S 32°30'E
Durban 29°52'S 31°00'E
Eshowe 28°53'S 31°28'E
Muden 28°58'S 30°22'E
Ngome Forest 27°48'S 31°25'E
Ngoya Forest, Mtunzini Distr. 28°52'S 31°43'E
Nkandla Forest 28°43'S 31°08'E
Weenen 28°51'S 30°05'E
Mpumalanga
Barberton 25°47'S 31°03'E
Blyde River Nature Reserve 24°15'S 30°50'E
Fourteen Streams, Barberton Distr. 25°47'S 31°08'E
Krokodilpoort Mts., 28 km SE Nelspruit 25°34'S 31°13'E
Malelane 25°29'S 31°31'E
Skukuza 25°00'S 31°35'E
Gauteng
Pretoria 25°43'S 28°11'E
Suikerbosrand Nature Reserve 26°30'S 28°15'E
Northern Province
Limburg, Potgietersrus Distr. 23°48'S 28°54'E
Louis Trichardt 23°02'S 29°54'E
Mutale, Venda 22°27'S 30°05'E
Naboomspruit 24°30'S 28°43'E
Penge, Lydenburg Distr. 24°22'S 30°18'E
Percy Fyfe Nature Reserve, Potgietersrus Distr. 24°03'S 29°10'E
Tshakoma, Zoutpansberg 23°03'S 30°18'E
Waterberg c. 24°00'S 28°00'E
Wylies Poort 22°53'S 29°56'E
SWAZILAND
Bulungu Mts. 26°40'S 31°32'E
Jilobi Forest, Lubombo Mts. 26°33'S 31°58'E
Mlawula 26°11'S 32°01'E
Mpisi 26°25'S 31°32'E
ZIMBABWE
Christon Bank 17°36'S 31°00'E
Laurenceville, Vumba Mts. 19°05'S 32°48'E
MOZAMBIQUE
Bela Vista 26°20'S 32°40'E
ZAMBIA
Solwezi 12°10'S 26°26'E
References
BRETHERTON, R. F., GOATER, B. & LORIMER, R. I., 1983. Noctuidae: Cuculliinae to Hypeninae. Pp. 36-413 in HEATH, J. (ed.), The Moths and Butterflies of Great Britain and Ireland vol. 10. Harley Books, Colchester.
GAEDE, M., 1934. Eulenartige Nachtfalter: Acronyctinae. In: SEITZ, A., ed., Die Gross-Schmetterlinge der Erde, Vol. 15: pp. 30-32. Alfred Kernen, Stuttgart.
HAMPSON, G. F., 1911. Descriptions of new genera and species of Syntomidae, Arctiadae, Agaristidae and Noctuidae. Annals and Magazine of natural History (8) 8: 394445.
JANSE, A. J. T., 1937-1939. The Moths of South Africa 3: Cymatophoridae, Callidulidae and Noctuidae (partim). E.P. and Commercial Printing Co., Durban.
KITCHING, I. J. & RAWLINS, J. E., 1999. 19. The Noctuoidea. In: KRISTENSEN, N.P., Volume Editor, Lepidoptera, Moths and Butterflies, Volume 1: Evolution, Systematics and Biogeography; Part 35, pp. 355-401 in: Handbook of Zoology, Volume IV Arthropoda: Insecta. Walter de Gruyter, Berlin and New York.
KOCH, M., 1984. Wir bestimmen Schmetterlinge. 792 pp. Neumann, Neudamm.
LOW, A. B. & REBELO, A. G., 1998. Vegetation of South Africa, Lesotho and Swaziland. 85 pp. and map. Department of Environmental Affairs & Tourism, Pretoria.
NYE, I. W. B., 1975. The generic names of moths of the world. Vol. 1, Noctuoidea (part): Noctuidae, Agaristidae, and Nolidae. British Museum (Natural History), London. 568 pp.
POOLE, R. W., 1989. Lepidopterorum Catalogus (new series), Fascicle 118, Noctuidae. Part 1-3. E.J. Brill/Flora and Fauna Publications, Leiden.
ROBINSON, G. S., 1976. The preparation of slides of Lepidoptera genitalia with special reference to the Microlepidoptera. Entomologists Gazette 27: 127-132.
SCOBLE, M. J., 1992. The Lepidoptera - form, function and diversity. Natural History Museum Publications & Oxford University Press. 404 pp.
Legends
Figs 1-4, adults. 1, Acronicta transvalica; 2, A. vumbae; 3, A. niveogrisea; 4, A. silvicola. Scale bar in mm.
Figs 5, 6, male genitalia. 5, Acronicta niveogrisea; 6, A. silvicola. Scale bar = 1 mm.
Figs 7, 8, female genitalia. 7, Acronicta niveogrisea; 8, A. silvicola. Scale bar = 1 mm.
Map 1. Geographical distribution of Acronicta transvalica.
Map 2. Geographical distribution of Acronicta niveogrisea (), A. silvicola () and A. vumbae ().